Home


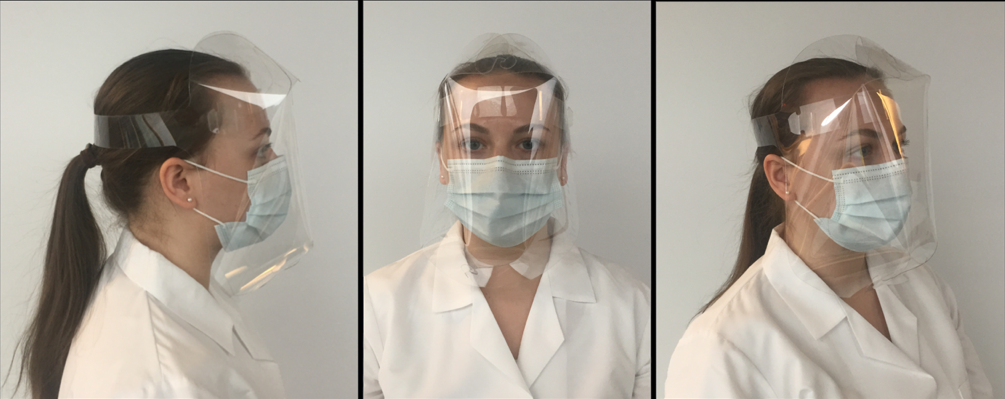
FDA Emergency Use Authorization: The manufacture of this face shield design is a humanitarian response to the COVID-19 pandemic.
-
•This product has been authorized by the FDA under an EUA for use by healthcare providers as personal protective equipment.
-
•This product is authorized for the duration of the declaration under Section 564(b)(1) of the Act, 21 USC 360bbb-3(b)(1).
As with many other face shield designs, this product is not intended for:
-
•Surgical use, where direct sterile field contact is expected
-
•Uses where significant amounts of exposure to hazardous fluid is expected
-
•Situations where level 3 or 4 protection is required (moderate to high barrier protection)
-
•Situations with a possibility of exposure to high heat, excessive humidity, or flammable gas
Body Containing Materials:
-
•Anti-fogging Food-grade 0.012” Poly-Ethylene Terephthalate (PET)




Made in the USA at an FDA-approved manufacturing facility